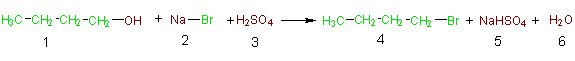
Developed by Paul Anastas and John Warner in 1998*, the following list outlines a a framework for making a greener chemical, process, or product.
Click on the tabs to reveal articles about each principle. These articles were originally developed for The Nexus Blog.
It is better to prevent waste than to treat or clean up waste after it has been created. Contributed by Berkeley W. Cue, Jr., Ph.D., BWC Pharma Consulting, LLC. In their publication “Green Chemistry, Theory and Practice” in 1998, Anastas and Warner introduced their 12 principles. My view is the first principle, often referred to as the prevention principle, is the most important and the other principles are the “how to’s” to achieve it An often-used measure of waste is the E-factor, described by Roger Sheldon, which relates the weight of waste coproduced to the weight of the desired product. More recently, the ACS Green Chemistry Institute Pharmaceutical Roundtable (ACS GCIPR) has favored process mass intensity, which expresses a ratio of the weights of all materials (water, organic solvents, raw materials, reagents, process aids) used to the weight of the active drug ingredient (API) produced. This is an important roundtable focus because of the historically large amount of waste coproduced during drug manufacturing—more than 100 kilos per kilo of API in many cases. However, when companies apply green chemistry principles to the design of the API process, dramatic reductions in waste are often achieved, sometimes as much as ten-fold. So, it is important to extend the impressive results achieved by the ACS GCIPR to all parts of the drug industry, especially the biopharma and generic sectors, as well as to other sectors of the chemical enterprise where synthetic chemistry is used to produce their products. More Resources & Examples: Process Mass Intensity Tool 2012 PGCCA Winner: Codexis, Inc. and Professor Yi Tang, University of California, Los Angeles “An Efficient Biocatalytic Process to Manufacture Simvastatin” 2002 PGCCA Winner: Pfizer, Inc. “Green Chemistry in the Redesign of the Sertraline Process” Pharma Strives for Green Goals, Stephen K. Ritter, Chemical & Engineering News, 90(22), May 28, 2012. Articles Cited: The E Factor: fifteen years on; R.A. Sheldon; Green Chem. 2007, (9), pp 1273-1283, DOI: 10.1039/B713736M Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes; Concepcion Jimenez-Gonzalez, Celia S. Ponder, Quirinus B. Broxterman, and Julie B. Manley; Org. Process Res. Dev., 2011, 15 (4), pp 912–917, DOI: 10.1021/op200097d.
Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Contributed by Michael Cann, Ph.D., Professor of Chemistry, University of Scranton The second principle of green chemistry can be simply stated as the “atom economy” of a reaction. Atom economy, which was developed by Barry Trost 1 , asks the question “what atoms of the reactants are incorporated into the final desired product(s) and what atoms are wasted?” Traditionally, the efficiency of a reaction has been measured by calculating the percent yield. Let us assume that the following substitution reaction gives 100% yield. While this is admirable, we can shed more light on the efficiency of a reaction by calculating the “percent atom economy” as follows:
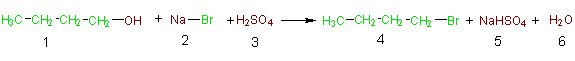
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100 = (137/275) X 100 = 50% The percent atom economy is simply the formula weight of the desired product(s) (compound 4, 137 g/mol) divided by the sum of the formula weights of all the reactants (275 g/mol), which gives 50% in this case. Simply put, even if our percent yield is 100%, only half the mass of the reactants atoms are incorporated in the desired product while the other half is wasted in unwanted by-products. Imagine telling your mom you baked a cake and threw away half the ingredients! Thus chemists must not only strive to achieve maximum percent yield, but also design syntheses that maximize the incorporation of the atoms of the reactants into the desired product. Principle #2 deals with the reactants. However, as those of us who have run a chemical reaction know, we usually use other materials such as solvents and separating agents during a synthesis. These materials usually make up the bulk of the material input, and thus we must also account for the waste that is produced from them. Stay “tuned” as you will see these discussed in subsequent Principles of Green Chemistry. More Resources & Examples: Atom Economy: A Measure of the Efficiency of a Reaction. Michael C. Cann and Marc E. Connelly; Real-World Cases in Green Chemistry; ACS, Washington, 2000. 1998 PGCCA Winner: Professor Barry M. Trost of Stanford University, "The Development of the Concept of Atom Economy." Articles Cited: 1. The Atom Economy-A Search for Synthetic Efficiency; Barry M. Trost; Science 1991, (254), pp 1471-1477.
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment. Contributed by David J. C. Constable, Ph.D., Director, ACS Green Chemistry Institute ® When you think about it, this is a two-part principle divided by the first two words, “wherever practicable.” Saying those two words implies that it may not be practical or possible to avoid using substances that are toxic, and this is, if you will, the get out of jail card most chemists use to try to avoid applying this principle to their work. Let’s face it; chemists use toxic substances all the time because reactive chemicals afford reactions that are kinetically and thermodynamically favorable. And unless—and until—replacement chemicals along with new synthetic protocols are developed, inherently toxic materials will continue to be used. But it’s easier to say that it isn’t practicable and dispense with any thought about the chemical choices that are made. It’s not that adhering to this principle is particularly difficult to do; it’s more that chemists are disinterested in doing it. For the synthetic organic chemist, effecting a successful chemical transformation in a new way or with a new molecule or in a new order is what matters. I have heard such arguments, as “all the other stuff in the flask is just there to make the transformation possible so it really doesn’t matter,” or “you have to be realistic and focus on the science.” Saying these things implies that the only science that matters is activating a carbon atom to functionalize it, or adding a ligand to a catalyst, etc., etc. This principle is asking chemists to broaden their definition of what constitutes good science. What many have shown over and over again is that toxicity and the attendant hazard and risk associated with a chemical reaction is directly related to all the other “stuff” in a flask. In fact, the chemistry or chemical transformation in a synthesis generally impacts the overall toxicity profile (and most other measures of sustainability and green) of a product or process the least, except in those cases where we deliberately are producing a molecule that is toxic or biologically active by design. That is certainly the case for many molecules that are synthesized as in the pharmaceutical or agriculture chemical business—the molecules are toxic and/or have other effects on living organisms by design. The chemicals and materials used in effecting chemical transformations matter and chemists need to pay more attention to the choices they make about what goes into the flask. It’s easy to discount all the other “stuff” and focus all our energy on the synthetic pathway that delivers the desired product. But when we ignore all the other “stuff,” we pay a high price and it’s a price we need to stop paying. Occasionally, chemists do produce molecules that have toxic or other hazardous effects, and the next principle will have something to say about designing safer molecules.
Chemical products should be designed to preserve efficacy of function while reducing toxicity.
Contributed by Nicholas D. Anastas, Ph.D., U.S. Environmental Protection Agency- New England Minimizing toxicity, while simultaneously maintaining function and efficacy, may be one of the most challenging aspects of designing safer products and processes. Achieving this goal requires an understanding of not only chemistry but also of the principles of toxicology and environmental science. Highly reactive chemicals are often used by chemists to manufacture products because they are quite valuable at affecting molecular transformations. However, they are also more likely to react with unintended biological targets, human and ecological, resulting in unwanted adverse effects. Without understanding the fundamental structure hazard relationship, even the most skilled molecular magician enters the challenge lacking a complete toolkit. Mastering the art and science of toxicology requires innovative approaches to chemical characterization that state that hazard is a design flaw and must be addressed at the genesis of molecular design. The intrinsic hazard of elements and molecules is a fundamental chemical property that must be characterized, evaluated and managed as part of a systems-based strategy for chemical design. Now is the ideal time to develop a comprehensive and cooperative effort between toxicologists and chemists, focused on training the next generation of scientists to design safer chemicals in a truly holistic and trans-disciplinary manner through innovative curricular advancements. The field of toxicology is evolving rapidly, incorporating and applying the advancements made in molecular biology to reveal the mechanisms of toxicity. Elucidation of these pathways serve as the starting point for articulating design rules that are required by chemists to guide their choices in a quest to make safer chemicals. We are at the dawn of a new sunrise, poised to illuminate the path forward to a safer, healthier and more sustainable world. More Resources and Examples Anastas, N. Green Toxicology, 2012 in: Green Techniques for Organic Synthesis and Medicinal Chemistry, W. Zhang and B. Cue, eds., J Wiley. Anastas, N.D. and J.C. Warner. 2005. Incorporating Hazard Reduction as a Design Criterion in Green Chemistry, Chem. Health. Safety, March/April, 3-15. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes, 2009, A. Lapkin and D. Constable eds., J. Wiley. Green Chemistry Education: Changing the Course of Chemistry, 2009, ACS Symposium Series 1011, P.T. Anastas, I. Levy and K.E. Parent, eds. J. Wiley Designing Safer Chemicals, 1996, S. DeVito and R. Garrett eds., ACS Symposium Series 640. US EPA, 2013, http://epa.gov/ncct/Tox21/ (accessed 3/3/13) Disclaimer: Although these references are given to provide additional information that may be useful or interesting, EPA is not responsible for, and cannot attest to the accuracy of, the content of these articles.
The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and, innocuous when used. Dr. Concepcíon (Conchita) Jiménez-González, Director, Operational Sustainability, GlaxoSmithKline It was a green chemistry conference and the very famous synthetic chemist had just received a question about why he had chosen a solvent that was without question a very poor choice. You have to be realistic, chemists know intuitively what's best, and solvents don't matter. It's the chemistry that counts. I've heard this kind of remark repeatedly over many years, despite the fact that it goes against the spirit and letter of Principle 5. Solvents and mass separation agents of all kinds matter a lot to the chemistry not to mention the chemical process and the overall "greenness" of the reaction. In many cases, reactions wouldn't proceed without solvents and/or mass separation agents. To say that they don't matter, or that it's only the chemistry that counts is not just a logical fallacy, it's chemically incorrect. Solvents and separation agents provide for mass and energy transfer and without this, many reactions will not proceed. It has also been shown that solvents account for 50 – 80 percent of the mass in a standard batch chemical operation, depending on whether you include water or you don't. Moreover, solvents account for about 75% of the cumulative life cycle environmental impacts of a standard batch chemical operation. Solvents and mass separation agents also drive most of the energy consumption in a process. Think about it for a moment. Solvents are alternately heated, distilled, cooled, pumped, mixed, distilled under vacuum, filtered, etc. And that's before they may or may not be recycled. If they're not recycled, they are often incinerated. Solvents are also the major contributors to the overall toxicity profile and because of that, compose the majority of the materials of concern associated with a process. On average, they contribute the greatest concern for process safety issues because they are flammable and volatile, or under the right conditions, explosive. They also generally drive workers to don personal protective equipment of one kind or another. We will always need solvents, and with many things in chemical processes, it's a matter of impact trading. Optimize a solvent according to one green metric and many times, there are three others that don't look so good. The object is to choose solvents that make sense chemically, reduce the energy requirements, have the least toxicity, have the fewest life cycle environmental impacts and don't have major safety impacts. Solvents and separation agents do matter and despite one or more famous synthetic organic chemists may think. It is possible to make better choices, and that is what application of this principle should promote.
Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure. By Dr. David Constable, Director, ACS Green Chemistry Institute ® In recent years I've begun to talk about the green chemistry and engineering's "forgotten principles," and Design for Energy Efficiency is one of them. Amongst synthetic organic chemists, no consideration is given to temperature or pressure. The chemist just follows a protocol to get a reaction to go to completion and to separate the desired product at as high a yield as possible. Energy, from the chemist’s perspective, is irrelevant and for all intents and purposes, free. Just put the plug in the wall or the heating coil around the flask, or get the liquid nitrogen out of the dewar. For those that do think about energy, most if not all the attention that energy gets from chemists is devoted to heating, cooling, separations, electrochemistry, pumping and reluctantly, to calculations related to thermodynamics (e.g., Gibbs Free Energy). The attention is not in minimizing or considering where energy comes from or if it matters what form is used, it's just a given that we need to heat or cool or shove electrons into the reaction to make or break bonds. In reflecting on my own training as a chemist, I never was asked to convert any heating, cooling, pumping or electrochemical requirements to a cost for electricity, steam or some other utility. That may be done in chemical engineering, but not in chemistry. Energy is a key issue for the 21st century. A majority of the energy that is produced is based, and will continue to be based on fossil fuels. And most of the energy that is delivered to the point of use is lost in conversion and transmission. What this means is that if you look at the life cycle of energy production, and you look at how much energy is actually available for useful work at the point of need, it is less than 1 or 2 percent of the energy that was originally available in the fossil fuel. It is also true that most fossil fuel energy is used for transportation services of one kind or another and the second biggest use is in space heating and cooling. There are a tremendous number of opportunities for chemists to change this energy use profile, but it is my experience that very few chemists see themselves as being a part of either transportation or the built environment. If you think about where most chemists are trained around energy, and certainly chemical engineers are, it's around ∆H in the Gibbs Free Energy equation. Heats of formation, heats of vaporization, enthalpy, exothermic reactions, etc; these are what we think about. The interesting thing is that nature largely works with ∆S and weak forces of interaction. You don’t see a tree doing photosynthesis at reflux using a solvent, or a cell membrane is not extruded at the melt temperature of something like polystyrene. There is so much more to energy and engaging chemists in thinking about energy than asking them to run reactions at ambient temperature and pressure. Reactions themselves are rarely where a majority of energy is used; most is used in solvent removal to set up for the next reaction, or to remove one solvent and replace it with another, or to isolate the desired product, or to remove impurities. Apart from hydrogenations or reactions that are oxygen or moisture sensitive, most reactions are done at atmospheric pressure. This doesn't mean that energy isn't important, it is just important in areas where most chemists are not focused. Once again, thinking about more than one part of the reaction or the process during the design of a new molecule is critical not only from the standpoint of energy, but also from many different angles. Energy—like thinking about how to arrange a synthesis to have the fewest number of steps, or use the lowest cost starting materials or any other aspect of interest to the synthetic or process chemist—is just another design parameter. Historically it has not been seen as that, but we can no longer afford to design new molecules in the absence of a detailed and extended consideration of how energy will be used.
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. By Dr. Richard Wool, Professor of Chemical and Biomolecular Engineering and Director of the Affordable Composites from Renewable Materials program, University of Delaware. The concept of making all our future fuels, chemicals and materials from feedstocks that never deplete is an interesting concept which at first glance seems impracticable. Mankind currently removes fossil fuels, coal, oil and natural gas from the ground and extracts minerals for profit until they are exhausted. In particular, our fossil fuels for carbon-based chemicals and materials are being rapidly depleted in a predictable manner with the expected rise of global populations and expanding energy intensive economies on several continents. The impacts on human health and the environment are significant and present major challenges for our scientists and leaders in the next 50 years. Can we address these global problems by using Green Chemistry Principal #7? Yes, we will get our feedstock, as if by magic, from “thin air” and it will be renewable. The carbon in the air is in the form of carbon dioxide CO2 and methane CH4 and is removed by photosynthetic processes powered by the sun to form plants, trees, crops, algae, etc., which collectively we call “biomass”. Nature produces about 170 billion tons of plant biomass annually, of which we currently use about 3.5 percent for human needs. It is estimated that about 40 billion tons of biomass, or about 25 percent of the annual production, would be required to completely generate a bio-based economy. The technical challenge in the use of such renewable feedstocks is to develop low energy, non-toxic pathways to convert the biomass to useful chemicals in a manner that does not generate more carbon than is being removed from “thin air”; the difference between C(in) from the air, and C(out) from the energy used, is the carbon footprint ΔC. Ideally, when using Principal #7, all carbon footprints by design should be positive such that C(in) >> C(out). This leads in a natural way to the reduction of global warming gasses impacting our current climate change. We should also insure that the new chemicals and materials derived from renewable resources are non-toxic or injurious to human health and the biosphere. In 2002, the U.S. Department of Energy in their Vision for Bioenergy and Bio-based Products in the United States stated: “By 2030, a well-established, economically viable, bioenergy, and bio-based products industry is expected to create new economic opportunities for rural America [globalization through localization], protect and enhance the environment, strengthen the U.S. energy independence, provide economic security, and deliver improved products to consumers.” In the past 10 years, significant advances have been made in the development of fuels, chemicals and materials from renewable feedstocks. These for example, have included biodiesel from plant oils and algae, bioethanol and butanol from sugars and lignocellulose, plastics, foams and thermosets from lignin and plant oils, and even electronic materials from chicken feathers. In terms of Green Chemistry Principal #7, our future is bright and laced with optimism due to the ongoing fruitful collaborations between several disciplines involving biotechnology, agronomy, toxicology, physics, engineering and others, where new fuels, chemicals and materials are being derived from renewable feedstock from “thin air” with minimal impact on human health and the environment. Additional Resource Vision for Bioenergy and Biobased Products in the United States - Updated 2006
Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
By Peter J. Dunn, Green Chemistry Lead, Pfizer One of the key principles of green chemistry is to reduce the use of derivatives and protecting groups in the synthesis of target molecules. One of the best ways of doing this is the use of enzymes. Enzymes are so specific that they can often react with one site of the molecule and leave the rest of the molecule alone and hence protecting groups are often not required. A great example of the use of enzymes to avoid protecting groups and clean up processes is the industrial synthesis of semi-synthetic antibiotics such as ampicillin and amoxicillin. In the first industrial synthesis Penicillin G (R=H) is first protected as its silyl ester [R = Si(Me)3] then reacted with phosphorus pentachloride at -40 o C to form the chlorimidate 1 subsequent hydrolysis gives the desired 6-APA from which semi-synthetic penicillins are manufactured.

(i) TMSCl then PCl5, PhNMe2, CH2Cl2, -40oC (ii) n-BuOH, -40oC, then H2O, 0oC (iii) Pen-acylase, water This synthesis has been largely replaced by a newer enzymatic process using pen-acylase. This synthesis occurs in water at just above room temperature. The new synthesis has many advantages from a green perspective one of which is that the silyl protecting group is not required. More than 10,000 metric tons of 6-APA is made every year and much of it by the greener enzymatic process so this is a fantastic example of Green Chemistry making a real difference. More Resources The Importance of Green Chemistry in Process Research and Development
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. Contributed by Roger A. Sheldon, Ph.D., Emeritus Professor of Biocatalysis and Organic Chemistry, Delft University of Technology and CEO of CLEA Technologies B.V. A primary goal of green chemistry is the minimization or preferably the elimination of waste in the manufacture of chemicals and allied products: “prevention is better than cure”. This necessitates a paradigm shift in the concept of efficiency in organic synthesis, from one that is focused on chemical yield to one that assigns value to minimization of waste. What is the cause of waste? The key lies in the concept of atom economy: “synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product”. In the reaction scheme we compare, for example, the reduction of a ketone to the corresponding secondary alcohol using sodium borohydride or molecular hydrogen as the reductant. Reduction with the former has an atom economy of 81% while reduction with the latter is 100% atom economic, that is everything ends up in the product and, in principle, there is no waste.

Unfortunately, hydrogen does not react with ketones to any extent under normal conditions. For this, we need a catalyst such as palladium-on-charcoal. A catalyst is defined as “a substance that changes the velocity of a reaction without itself being changed in the process”. It lowers the activation energy of the reaction but in so doing it is not consumed. This means that in principle at least, it can be used in small amounts and be recycled indefinitely, that is it doesn’t generate any waste. Moreover, molecular hydrogen is also the least expensive reductant and, for this reason, catalytic hydrogenations are widely applied in the petrochemical industry, where the use of other reductants is generally not economically viable. It is only in the last two decades, however, following the emergence of green chemistry, that catalysis has been widely applied in the pharmaceutical and fine chemical industries, with the goal of minimizing the enormous amounts of waste generated by the use of stoichiometric inorganic reagents. This involves the use of the full breadth of catalysis: heterogeneous, homogeneous, organocatalysts and, more recently, Nature’s own exquisite catalysts: enzymes. The latter are particularly effective at catalyzing highly selective processes with complex substrates under mild conditions and, hence, are finding broad applications in the pharmaceutical and allied industries. Moreover, they are expected to play an important role in the transition from a chemical industry based on non-renewable fossil resources to a more sustainable bio-based economy utilizing renewable biomass as the raw material, yet another noble goal of green chemistry.
More Resources and Examples R.A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007 (ISBN 978-3-527-30715-9) R.A. Sheldon, Fundamentals of green chemistry: efficiency in reaction design, Chem. Soc. Rev. 41 (2012) 1437-1451. R.A. Sheldon, E Factors, green chemistry and catalysis: An odyssey Chem. Commun. (2008) 3352-3365.
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment. Contributed by Rich Williams, Founder and President at Environmental Science & Green Chemistry Consulting, LLC Green chemistry practitioners aspire to optimize the commercial function of a chemical while minimizing its hazard and risk. Hazard, the capability to cause harm, is an inherent characteristic arising, like function, from a chemical’s stereochemistry (the content and arrangement of atoms). Green chemistry principles 3, 4, 5, and 12 guide designers to reduce the hazards of chemicals. Principle 10, however, guides the design of products that degrade after their commercial function in order to reduce risk or the probability of harm occurring. Risk is a function of both a molecule’s inherent hazard AND exposure – contact between a chemical and a species. Degradation can eliminate significant exposure, thereby minimizing risk regardless of the hazard of the chemical involved. Exposure to persistent chemicals can be significant as a result of global dispersion enabled by properties such as volatility or sorption to particles and partitioning into organisms based on properties such as fat solubility. Regulators have established criteria (half-lives in water, soil, air) that define persistence within frameworks used to identify chemicals as PBT (Persistent, Bioaccumulative, Toxic). A green chemistry objective is to design out molecular features responsible for hazardous characteristics and risk. Trade-offs, or alternative approaches, must be evaluated when the molecular features to be designed in for commercial function overlap with those to be designed out to reduce hazard and risk. Biodegradation, hydrolysis, and photolysis can be designed into chemical products. In the same way that mechanistic toxicology knowledge is essential to identify and design out molecular features that are the basis for hazards, an understanding of the mechanisms of degradation and persistence are required to design in chemical features that promote degradation and eliminate features that promote persistence. Many persistent compounds are extensively chlorinated. Halogens such as chlorine are electron withdrawing, thereby inhibiting the enzyme systems of microbes because aerobic microbial degradation favors electron rich structures. Prediction methods that can guide the design of molecular architecture expected to degrade include rules of thumb linking structural features to degradability or persistence, databases of existing knowledge, models that evaluate biodegradability or PBT attributes, and experimental testing. All of these tools can be adapted to individual chemical sectors and specific objectives. Understanding the anticipated release and transport pathways for a chemical informs the selection of an effective design strategy. Degradation must occur within the relevant environmental compartment(s) and at a meaningful rate. Domestic wastewater typically passes through a vigorous bioreactor within wastewater treatment plants (WWTP). The consumer product industry has designed molecules for removal within these bioreactors. In the early 1960’s, industry transitioned from non-biodegradable branched surfactants, which caused extensive foaming and other health problems in surface waters receiving WWTP effluent, to biodegradable linear alkyl benzene sulfonate based detergents – an approach to innovative design that continues today. Tools currently exist to enable the implementation of principle 10, but advances in mechanistic understandings linking molecular features to hazards and degradability will enable more comprehensive application of green chemistry to control hazard and risk. Effective communication across disciplines is also essential to provide designers with knowledge they can factor into the complexities of product design. Because of regulatory and business constraints, many product design decisions must be made relatively early. Predictive decision-making tools must provide confidence about hazard and risk in a way that is aligned with the timing and magnitude of development decisions, and most importantly, while there is still flexibility to alter a molecular design or product formulation.
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. Contributed by Douglas Raynie, Assistant Professor, Chemistry & Biochemistry, South Dakota State University Imagine driving down a busy highway in a car with all of the windows painted an opaque black. While that scenario many not seem realistic (or safe), what if you had a 360° camera and the sensors and technology being developed for self-driving cars? Now, the safety of your commute is more ensured. This description, while applied to automobiles, is illustrative of the 11th principle of green chemistry. Just as we need real-time feedback for driving safety, real-time feedback is essential in proper functioning chemical processes. Most chemists are familiar with laboratory analysis from their undergraduate training. But analysis can also be performed in-line, on-line, or at-line in a chemical plant, a subdiscipline known as process analytical chemistry. Such analysis can detect changes in process temperature or pH prior to a reaction going out of control, poisoning of catalysts can be determined, and other deleterious events can be detected before a major incident occurs. Process analysis is of such importance that the US Food and Drug Administration encourages such an approach for the manufacture, design, and control of pharmaceutical manufacturing. Since 1984, an industry-academic partnership, the Center for Process Analytical Chemistry, has promoted research into emerging techniques for process analytical chemistry. While the traditional roles of analytical chemistry also advance green chemistry goals, the effective application of process analytical chemistry directly contributes to the safe and efficient operation of chemical plants worldwide. Additional resource: Center for Process Analysis & Control
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires. Contributed by Shelly Bradley, Campus Chemical Compliance Director, Hendrix College; Dr. David C. Finster, Professor of Chemistry, Wittenberg University; and Dr. Tom Goodwin, Elbert L. Fausett Professor of Chemistry, Hendrix College Safety can be defined as the control of recognized hazards to achieve an acceptable level of risk. Green Chemistry Principle # 12 is known as the “Safety Principle”. It may be the most overlooked of the twelve principles, yet it is the logical outcome of many of the other principles. In fact, it is practically impossible to achieve the goals of Principle 12 without the implementation of at least one of the others. Since the very essence of green chemistry is to “… reduce or eliminate the use or generation of hazardous substances” there is an intrinsic connection to laboratory safety. While there are a few exceptions, the majority of the Green Chemistry Principles will result in a scenario that is also safer.

Under the umbrella of the Environmental Protection Agency (EPA), Green Chemistry’s primary focus is clearly to make the environment safer. Materials and processes that are safer for the environment also are likely to be safer for the general public. However, another population that benefits from green chemistry and is not often mentioned is workers. The manufacturing or laboratory worker is often the first in-line person to benefit from hazard reductions. The health and safety of workers are under the purview of the Occupational Safety and Health Administration (OSHA). In a recent news release, OSHA unveiled a chemical management system designed to increase worker safety. The Hierarchy of Safety Controls as highlighted in OSHA’s new Transitioning to Safer Chemicals Toolkit illustrates the difference between focusing on the control or hazard part of the safety definition. Traditional chemical safety models focus primarily on the control component of that definition. The graphic (adapted from OSHA) shows that the most effective means of increasing safety is eliminating the hazard component. Since the elimination of hazards is the basic tenet of Green Chemistry, this marriage of the ideas of Green Chemistry from both OSHA and EPA should have a synergistic impact on hazard reduction. Combining the forces of these two agencies toward a common goal may lead to conversations and changes that result in safer conditions for workers, a safer environment for the general public, and a safer planet for us all. References Cited: Manuele, F. A. Acceptable Risk, Professional Safety, 2010, 30-38 (accessed 11/22/2013) Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press; New York, 1998. US OSHA, OSHA releases new resources to better protect workers from hazardous chemicals, (accessed 11/22/2013) US OSHA, Transitioning to Safer Chemicals: A Toolkit for Employers and Workers, (accessed 11/22/2013) US OSHA, Why Transition to Safer Alternatives?, (accessed 11/22/2013)
*Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice, Oxford University Press: New York, 1998, p.30. By permission of Oxford University Press.